Abstract
Background: Regorafenib is a multikinase inhibitor that targets both angiogenic, stromal and oncogenic kinases including VEGFR-1, -2, -3, TIE-2, PDGFR- β, c-KIT, rRET, RAF-1, FGFR-1, BRAF and p38 MAPK. In vitro studies have demonstrated that regorafenib inhibits these clinically relevant kinases with half maximal inhibitory concentration (IC50) values between 4-180nM. As a result of regorafenib's broad inhibition of kinases, it may have the potential to overcome the limitations of more selective kinase inhibitors, and thus to have efficacy in a broad range of myeloid malignancies since these pathways are relevant in acute myeloid leukemia (AML), myelodysplastic syndromes, and myelofibrosis. Based on this rationale, we are currently conducting a multicenter, open-label phase I trial to assess the safety and tolerability of regorafenib in patients with advanced myeloid malignancies (NCT03042689). In addition, we are characterizing the mechanism of action of regorafenib in myeloid malignancies by conducting correlative studies (molecular and cellular biomarkers) in the clinical trial and using banked specimens.
Methods:
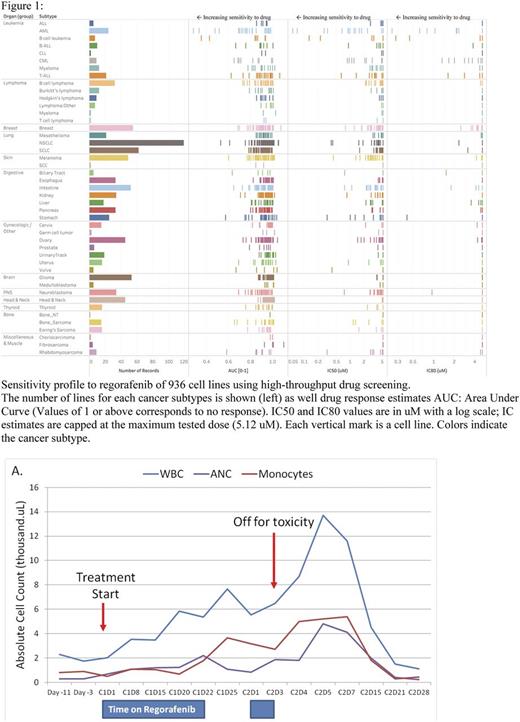
Cell Line Testing: Initial testing was performed utilizing a collection of greater than 1,000 human tumor cell lines representing the spectrum of common and rare types of adult and childhood cancers in the Benes Lab at Massachusetts General Hospital. Each cell line was treated with 9 doses 2-fold apart starting with a maximum dose of 5.12 uM (Minimum dose 20 nM; Range of 256-fold). The activity of the drug was estimated by fitting viability values (treated/DMSO control). The fitted values: Area Under the dose response Curve. A total of 95 leukemic lines were tested including 24 AML lines.
Genomic and Transcriptional Characterization- We performed genomic and transcriptional characterization of the leukemia cell lines tested to determine if there was a correlation between regorafenib response and mutational pattern. Genes tested included ASXL1, FLT3, Tp53, U2AF1, TET2, NRAS, MLL, BRAF, BMPR2, CDKn2A, Kd6A, Ptpn11, RB1 .
Results: High-throughput testing of regorafenib across multiple tumor types showed striking sensitivity of leukemia cell lines (Figure 1). AML lines were determined to be more sensitive than other cancer subtypes, p=0.00003 (2-tail Fisher's Exact Test). Chronic myeloid leukemia lines were also seen as sensitive compared to other subtypes likely due to the broad selectivity profile of regorafenib including activity against BCR-ABL. The genomic and transcriptional characterization of the cell lines tested thus far has not identified any associations between specific mutational profiles and response to regorafenib.
Of the patients treated on study so far in the clinical trial, one patient experienced a sustained and robust neutrophil count recovery after regorafenib (Figure 2A). Based on this initial finding, we examined whether differentiation of myeloid precursors may be a putative mechanism of action of regorafenib. We performed serial testing by flow cytometry to assess for differentiation. Testing in one patient suggested a change in the phenotype of the myeloid blasts on therapy from CD33+/CD13- to CD33+/CD13+ along with an increase in mature granulocytes (Figure 2B). We are currently collecting samples on study to determine if regorafenib leads to myeloid cell differentiation. In addition, we are assessing the pharmacodynamic effects of regorafenib by 1) evaluating the phosphorylation of targets such as VEGFR, FGFR, TIE-2, and cKIT, among others, and 2) measuring plasma levels of angiogenic and inflammatory biomarkers. Finally, we are measuring changes in specific markers of allele burden when applicable.
Conclusion: In summary, we are conducting a clinical study of regorafenib in myeloid malignancies, which will evaluate initial activity and characterize the mechanism of action and potential biomarkers of response to this drug in myeloid malignancies. Early results of a high throughput screen on multiple tumor types suggest AML is particularly sensitive to this agent. An updated biomarker analysis will be presented at the meeting.
Brunner: Celgene: Research Funding; Takeda: Research Funding. Duda: bayer: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal